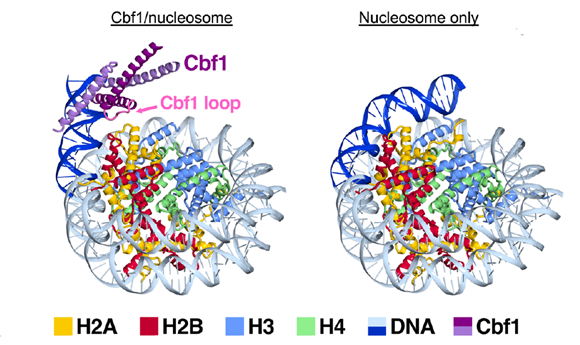
The mechanism of how pioneer factors invade into nucleosome is not clear. The long-term goal of this project is to develop a full mechanistic understanding of how pioneer factors access their nucleosomal binding sites, disrupt histone-DNA contacts, and activate gene expression. Towards this goal, we collaborated with Michael Poirer and Song Tan’s groups to conduct biochemical studies of pioneer factor-nucleosome interactions. Using single molecule assays, we found striking differences in nucleosome binding kinetics between pioneer factors vs canonical transcription factors. More specifically, we found that pioneer factors maintain high affinity to nucleosome through slow dissociation rates, which we named as “dissociation compensation” mechanism. A Cryo-EM structure of a pioneer factor, Cbf1, in complex with the nucleosome reveals that the Cbf1 DNA binding domain can electrostatically interact with exposed histone residues, which can potentially explain its slowed dissociation rate from nucleosome in comparison to naked DNA. In vivo studies show that this enhanced binding provided by the Cbf1 HLH region enables nucleosome invasion and ensuing repositioning.
Related publication:
- Basic helix-loop-helix pioneer factors interact with the histone octamer to invade nucleosomes and generate nucleosome depleted regions.
Donovan BT, Chen HY, Eek P, Meng ZY, Jipa C, Tan S†, Bai L†, Poirier MG† (2022)
BioRxiv. doi: https://doi.org/10.1101/2022.03.17.484790
- Dissociation rate compensation mechanism for budding yeast pioneer transcription factors
Donovan BT, Chen HY, Jipa C, Bai L, & Poirier MG (2019)
Elife, pii: e43008.


Leave A Comment